Which of the following solutions is the most dilute. The solution with 15 grams of honey and 100 mL of water.
Difference Between Dilute And Concentrated Solution Dilute Solution Vs Concentrated Solution
The solution with 5 grams of honey and 100 mL of water.
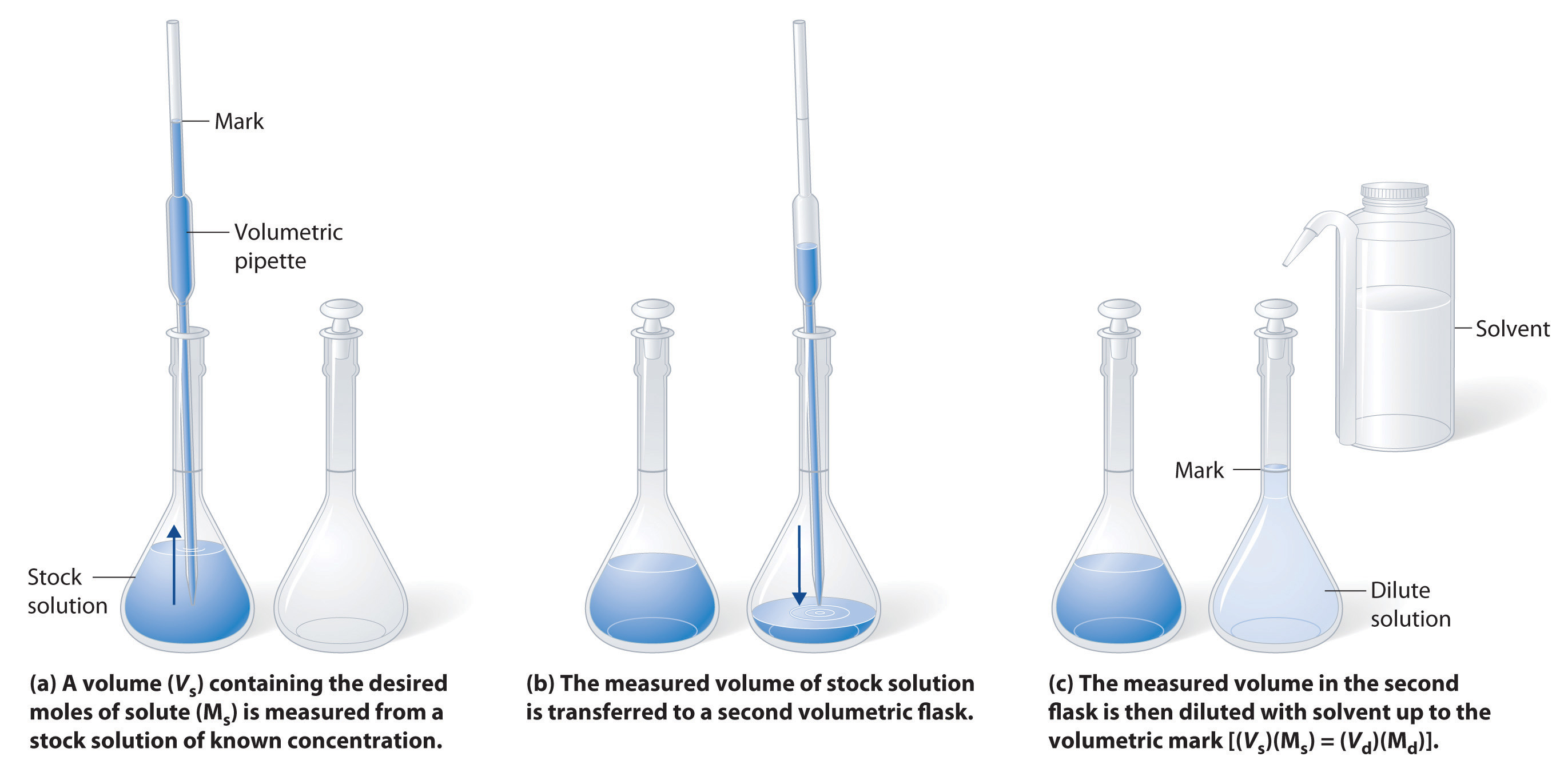
. CThe solution with 15 grams of honey and 100 mL of water. 02 liter of water with 2 grams of sugar B. To achieve a dilute solution more solvent is simply added without adding any more solute into the original mixture.
Correct answer - Which of the following solutions is the most dilute. The definition of dissolve is. They all have the same concentration.
X-2y2 y -2x5 a. A dilute solution has a low concentration of the solute compared to the solvent. 023 mol L e.
Aone liter of water with 1 gram of sugar b. One liter of water with 5 grams of sugar d. 1 liter of water with 10 grams of sugar E.
One liter of water with 2 grams of sugar. AThe solution with 10 grams of honey and 100 mL of water. After entering the required values the molar dilution calculator.
48 g1 H 2 S O 4 is the most dilute. 001 10 by volume 0001 M 1 ppm. The reaction is Bi3 H2PO4-.
Which of the following solutions is the most dilute. Particles from a mixture mixing together but then eventually settling down to the bottom. 01 liter of water with 1 gram of sugar B02 liter of water with 2 grams of sugar a c05 liter of water with 5 grams of sugar D.
Experts are tested by Chegg as specialists in their subject area. Infinitely many solutions b. The fused mass was dissolved in dilute acid following which the Bi3 was titrated with 2736mL of 003369M NaH2PO4.
M 1 denotes the concentration of the original solution and V1 denotes the volume of the original solution. First enter the value of the Initial Concentration and choose the unit of measurement from the drop-down menu. We review their content and use your feedback to keep the quality high.
Solution for Question 6 Which of the following solutions is the most dilute. If we order them from the most diluted to the most concentrated we get. See the answer See the answer done loading.
When calculating dilution factors it is important that the units for both volume and concentration are the same for both sides of the equation. 01 liter of water with 1 gram of sugar C. The solution with 10 grams of honey and 100 mL of water.
D e a b c. O 075 M NaOH O 12 M NAOH O 42 M NAOH O 16 M NACH. The most dilute would be the one having the less number of moles of solute per liters of solution that is solution d or e which have the same concentration.
They all have the same concentration. Here are the steps to follow for this solution calculator. Chemistry questions and answers.
25 mol L d. Which of the following solutions is more dilute. One solution chemistry.
For it normality N E S 49 48 098 N While 5 H 2 S O 2 1 50 g H 2 S O 4 102 N H 2 S O 4 and 05 M H 2 S O 4 10 N H 2 S O 4. Who are the experts. A dilute perchloric acid solution was standardized by dissolving 02445 g of primary standard sodium carbonate in 50 mL of the acid boiling to eliminate CO2 and back-titrating with 413mL of dilute NaOH.
Two or more substances mixing completely so that it appears as one. How many solutions does this system have. Which of the following solutions is the most dilute.
05 liter of water with 5 grams of sugar D. This can be expressed as molL or M. Then enter the value of the Initial Volume and choose the unit of measurement from the drop-down menu.
The opposite of a dilute solution is a concentrated solution which has high levels of solute in the mixture. M 2 represents the concentration of the diluted solution and V2 represents the final volume of the diluted solution. BThe solution with 5 grams of honey and 100 mL of water.
The solution with 20grams of honey and 100 mL of water. Two or more substances that are not able to mix completely. Which of the following solutions is the most dilute.
One liter of water with 10 grams of sugar c. Which of the following solutions is more dilute. 023 mol L.

8 1 Concentrations Of Solutions Chemistry Libretexts

What Is Dilute Solution Chemistry Youtube
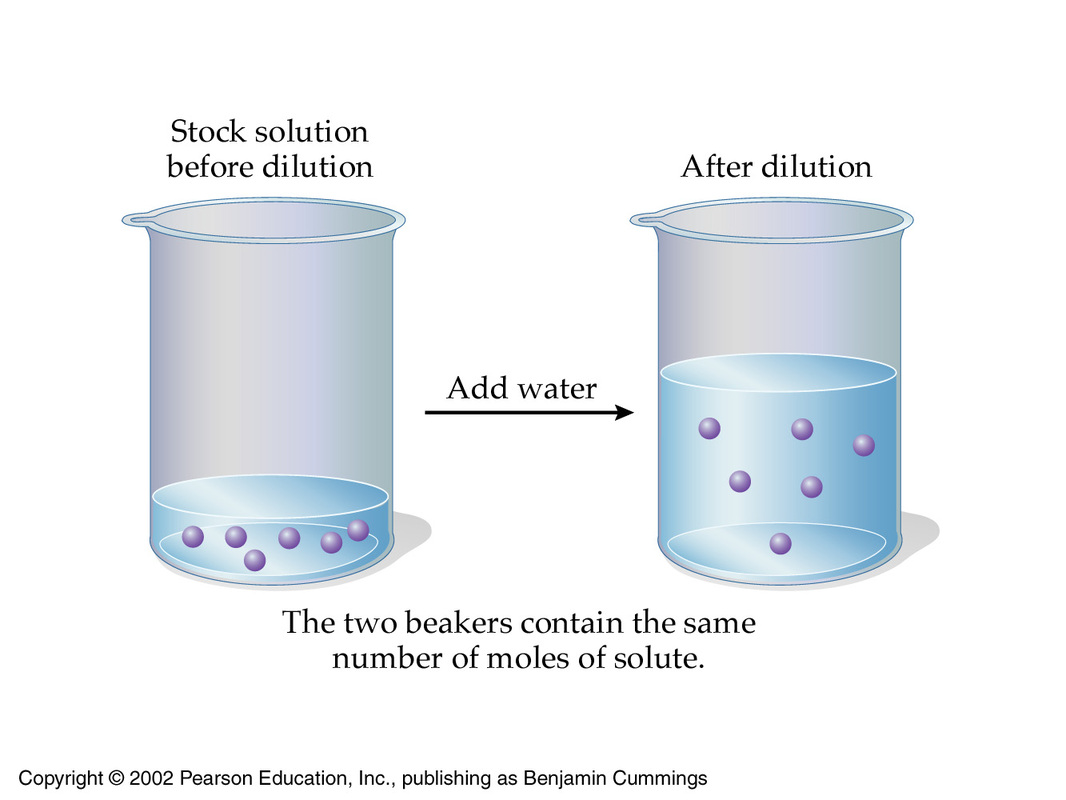
Ml Of Solution Has A Concentration Of 3 0 M How Much Water Needs To Be Added To Dilute The Solution To 1 9 M Socratic

0 Comments